T cell depletion
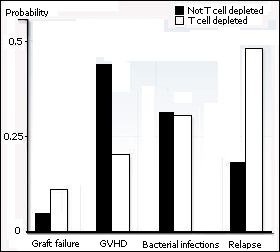 |
| Cumulative probability of transplant-related
complications after non-T-cell depleted and T-cell depleted HLA
identical sibling transplants. |
|
The removal of T cells from the donor graft reduces the incidence of
GVHD by limiting the number of alloreactive T cells capable of attacking the patient's tissues. T cells can be removed from the graft (in vitro) by physical separation or by treatment with T cell specific antibodies in combination with complement. A physical separation of the T cells and HSC can be achieved for example by agglutinating the T cells with lectins or by using specialized centrifugation techniques (elutriation). An alternative is to infuse only purified HSC.
T cell depletion can also be performed in vivo by injection of T cell specific antibodies (ATG, OKT3, Campath, etc.), which in combination with the patient’s complement will eliminate the T cells.
While reducing the frequency and severity of GVHD, T cell depletion increases
the risk of a leukemic relapse. This can be explained by the fact that the allogeneic
T cells recognize not only the tissues of the patient as foreign (negative side effect
-> GVHD) but also his leukemic cells (beneficial effect or graft-versus-leukemia effect (GVL). Basically, every treatment instigated to prevent GVHD by diminishing the activity of the allogeneic donor T cells will augment the risk of relapse of the original hematological malignancy. When a relapse occurs after transplantation, it is possible to take advantage of the GVL effect by infusion of fresh donor T cells. This treatment is called donor lymphocyte infusion (DLI).
In addition, T cell depletion increases the risk of rejection. Because donor T cell play a role in neutralizing/eradicating the patients immune/hematopoietic system, they help making space for the transfused HSC. Without T cell depletion, the risk of rejection is below 1%, after T cell depletion the risk remains low but increases to 1-3%.
|


 Print
Print